Explain How Electrons Can Produce Different Color Light
When an electron temporarily occupies an energy state greater than its ground state it is in an excited state. Fluorescent dyes absorb UV or blue light and use the energy absorbed to emit photons of different colors.

Color And Oxidation State Overview Examples Expii
Must be released by an electron in a mercury atom to produce a photon of this.

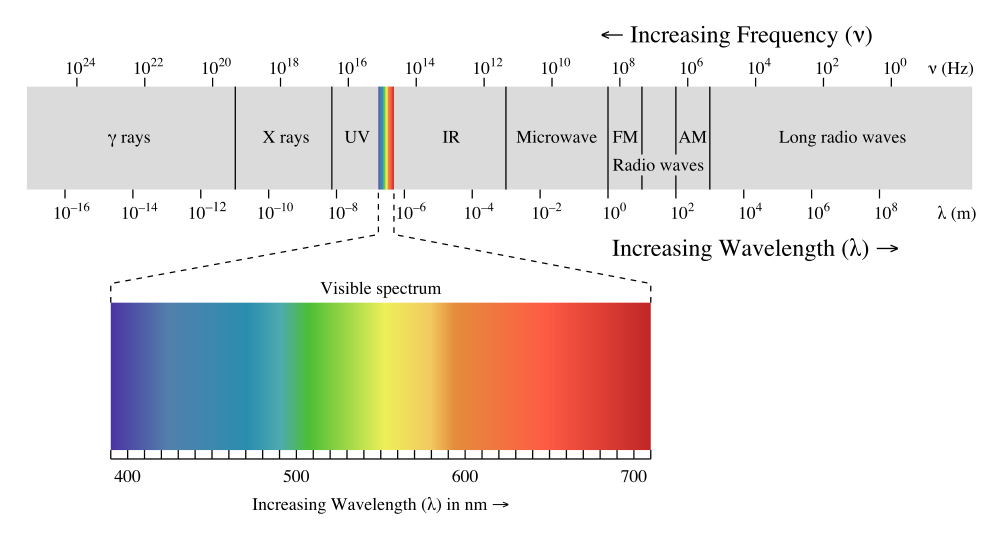
. Each electron in an atom has a specific amount of energy. Light is produced through a phenomenon known as electromagnetic radiation which is composed of both a magnetic and an electrical component. Light or visible light is merely one small part of the overall electromagnetic spectrum.
Thedifferent lights being emitted depends on their wavelength. The Bohr model of the atom established the existence of a positive nucleus surrounded by electrons in specific energy levels. Electrons must be heated to emit light.
Light that can be seen with the human eye also known as visible light has wavelengths between 750 and 380 nanometers. When heat was added to the elementsthe electrons absorbs the energy and moves to its excited state. The energy of light matches the energy gaps between electron shells.
Is known as the main energy shell in which the electrons can be. Since different elements have different electron configurations their electrons can give off different amounts of. There are multiple energy levels in all atoms that electrons can occupy so there are many possible colours even for the simplest single atoms.
The electrons inside atoms can only have certain energies so they have what are called energy levels. Each type of material emits a particular color of light. Electrons must be heated to emitted light.
Each element will produce a different colored flame. Explain what LED stands for. An electron can become excited if it is given extra energy such as if it absorbs a photon or packet of light or collides with a nearby atom or particle.
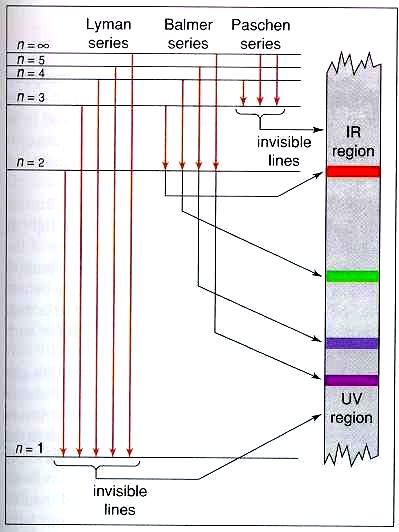
The color of the emitted light depends on its energy. Students can see that each gaseous element produces a different atomic spectrum. Depending on the element you put in the flame various different energies of.
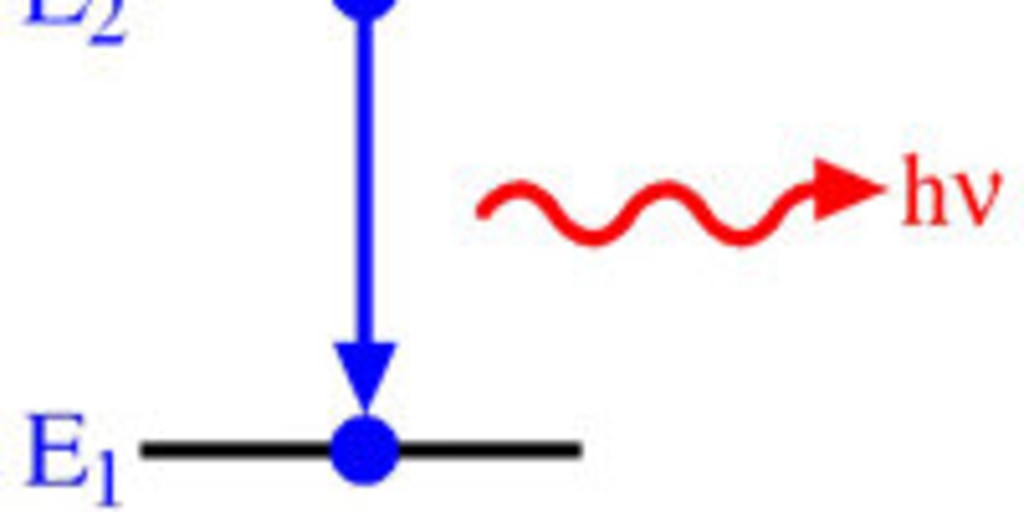
As they return to their ground state they emit visible light. Maybe they can absorb a certain amount of energy or twice that amount or two and a half times that amount changing. Electrons get to higher energy states and fall back to ground states emitting energy.
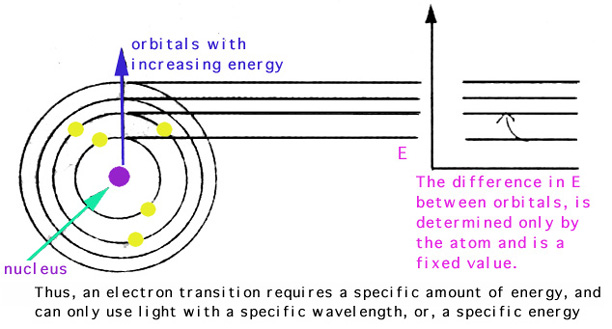
Electrons are arranged in energy levels shells and there are energy gaps between shells. The wavelength or color of light depends on the energy that is released ie. Colours come from electrons moving between shells.
Electrons must be in one shell and cannot be in between. Phosphorescence happens when photons are emitted after a delay for absorption. The entire spectrum includes gamma rays X-rays radio waves microwaves and infrared light.
The heat excites the element and caused reaction that produce different color of light. Different colors will emit depending the on wavelength. Once the electronstarts to fall back to a lower orbit it emits energy in the form of light.
Nanometers are small there are 1000000000 nanometers in 1 meter. Electrons can move from one shell to another in the right conditions. Remember ROY G BIV.
These excited electrons can fall back to lower energy levels their ground states releasing the excess energy in packages of light called photons. The electrons absorb the energy and move to their excited state. Blue light is more energetic than red light for example.
Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. To identify gaseous elements use the spectrum tube power supply the spectrum tubes and diffraction gratings or rainbow glasses. How Is Light Produced.
Therefore the temperature of the heat affects the color of the light. When the electron drops back it must release the same exact amount energy that it absorbed. When the heat was added to the elements.
The energy difference between the electrons two energy levels. The electrons jump from their ground state to a higher energy level. Once the electron starts to emit energy in the form of light.
The color of the light is connected to the location of the electrons and the affinity the outer-shell electrons have to the atomic nucleus. These stars hold fuel an oxidizing agent a binder and metal salts or metal oxides the source of the fireworks hues. Different parts of the rainbow are present for each element.
Like the distance between rungs of. Energy is released when electrons move from higher energy levels to lower ones visible light. As electrons move from higher-energy to lower-energy levels energy in.
The electron can return to its original energy ground state by releasing that energy as a photon light. Subsequently the light wave with that given frequency is absorbed by the object never again to be released in the form of light. Light Energy Each orbital has a specific energy associated with it.
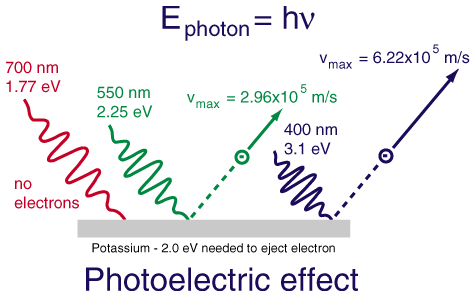
The energy of the emitted photon matches the energy lost by the electron and that energy determines the colour. Therefore depending on how much energy is released different colors can be given off. Different colours of light have different energies.
Releasing of this energy can come off in the form of light. When the elements were heated and the energy were absorbed² the electron released different colors of light As you can see from the lab² the electron ³star released certain lights with certain elements For example² Neon released the colors dark blue² green² and red While hydrogen released the colors blue² green² yellow² and red Provide scientific data from the lab. Show visitors the 3.
Because no two elements have the same set of energy levels different elements emit different colors of light. The color of the light that is produced depends on how far apart the excited energy is from the original energy. During its vibration the electrons interact with neighboring atoms in such a manner as to convert its vibrational energy into thermal energy.
Light emitting gives off light diode a material inside the bulb where electrons drop from a higher to a lower energy state giving off light in the process. The bluer the light and light comes in blobs called photons the more energy the photon has. Explain why different colors of light result from electron behavior in the atom.
24 5 Color And Magnetism Chemistry Libretexts

The Structures Of Plastoquinone A And Its Reduced Form Plastohydroquinone Or Plastoquinol Plastoquinone A Has Nine Isoprene U Chemistry Membrane Electrons

Science Poster Bundle Science Anchor Charts Science Poster 4th Grade Science

How Are Different Colored Fireworks Made Sciu

Tetryonics 40 06 The Source Electromotive Force Fields Of Physics Alchemic Symbols Physics Quantum Mechanics

47 Awesome Auroras To Delight Your Senses Webecoist Aurora Aurora Borealis Northern Lights Night Rainbow

Hid Lamps Are Mostly Used In Industrial Settings Retail Spaces Cars And Parking Lots They Produce A Very Cool Color And Are Not Retail Space Lamp Bulb Lamp

Constellation On Twitter Renewable Energy Geothermal Energy Sustainable Energy

Pin On High School Physical Sciences

Electric Current Concept Example Vector Illustration Electrical Circuit Diagram Free Electrons And Electrical Circuit Diagram Circuit Diagram Physics Poster

The Super Kamiokande Neutrino Observatory 3 300 Feet Underground A Neutrino Interaction With The Electrons Physics Experiments Physics Large Hadron Collider

Electron Energy And Light Spectra Youtube

How Are Different Colored Fireworks Made Sciu


Comments
Post a Comment